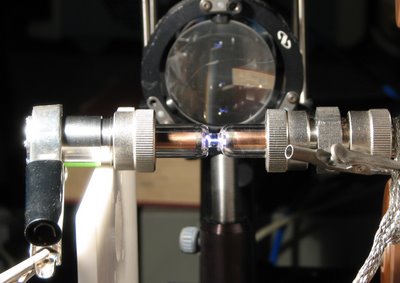
 I need to explain a few things. The smallerr diameter glass tube is about 5mm wide. It has an inner hollow diameter of 0.6mm, which is filled with hydrogen. Then along the cylindrical electrodes on either side we apply about 50,000 volts of voltage. This creates a huge electric field that breaks down (ionizes) the hydrogen atoms. This means that the field causes the electron that was until then orbiting the nucleus as if nothing was going on to be kicked out of its orbit and become free. Hence there is now a negative charge (electron) and also a positive charge left behind (proton). When this happens for most of the 10^19 hydrogen atoms per cubic centimeter, we have a plasma!
I need to explain a few things. The smallerr diameter glass tube is about 5mm wide. It has an inner hollow diameter of 0.6mm, which is filled with hydrogen. Then along the cylindrical electrodes on either side we apply about 50,000 volts of voltage. This creates a huge electric field that breaks down (ionizes) the hydrogen atoms. This means that the field causes the electron that was until then orbiting the nucleus as if nothing was going on to be kicked out of its orbit and become free. Hence there is now a negative charge (electron) and also a positive charge left behind (proton). When this happens for most of the 10^19 hydrogen atoms per cubic centimeter, we have a plasma!Now, this plasma glows. Many electrons are not fully freed but they just jump to a higher orbit, so they will return to their initial orbit after some (short) time. During that transition, they have to release back the energy they initially got from the 50,000 volts, and they will release it in the form of a photon, which is light. This is most of the light seen in this picture: it is electrons going up and down in their orbits when kicked out from the high voltage. Here is a more cool picture, without external lights:

All this luminus volume is filled with hydrogen plasma and it lights up. It also shows essentially the current that flows between the two electrodes of high voltage.
Now, what we need to know is how much plasma we create. We know that we put about 10^19 atoms there, but how many do actually get ionized, lose their electrons and become plasma? Ideally we would like all of them, but we don't know that. In order to figure it out, we measure the light emitted. When we look at the spectrum and the specific frequencies, we see lines. These lines correspond exactly to the orbit transitions of the electrons. You would expect the lines to be extremely narrow (corresponding exactly to the energy difference of the transition), but actually a guy name Stark won the Nobel Prize in the 1920's because he found out that these lines actually have a width! This is because once the plasma is created, you have electrons and protons (charges) flying around and that creates mini electric fields that alter these orbits a little bit. Since these fields are stronger the more electrons are there per unit volume, by measuring the lines' width we can tell how many electrons we have out there!
So far we measured 10^18 electrons per cubic centimeter, which is lower than we expected. We actually need 10^19 in order for our experiment at Brookhaven to work properly. In the next few days we will try to tune the parameters so that we can reach this number...